
UNIPROMID
IOPROMIDE INJECTION USP
Low Osmolar Non-Ionic Water Soluble Iodinated Contrast Media
- Contrast agent for intravascular administration opacifies vessels.
- Permitting radiographic visualization of internal structures until signicant hemodilutios occurs.
- low incidences of adverse reactions.
- Usage convenience.
| COMPOSITION | UNIPROMID 300 | UNIPROMID 370 |
|---|---|---|
| Iopromide | 62.34% | 76.88% |
| Iodine Concentration | 300mg/ml | 370mg/ml |
| pH | 7.25 +0.75 | 7.25 +0.75 |
| Viscosity (mPa*s) | ||
| @37℃ | 4.9 | 10 |
| @20℃ | 9.2 | 20 |
| Osmolality (mOsm/kg H2O) | ||
| @37℃ | 0.607 | 0.774 |
Comparative Osmolality
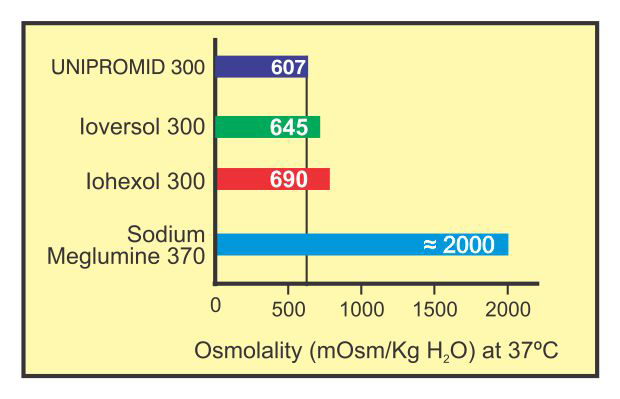
Lesser the osmotic pressure exerted by a contrast medium, better isthe tolerance. High osmolality is associated not only with pain and discomfort in injection but also with effects on the Haemodynamic System.
Comparative Viscosity
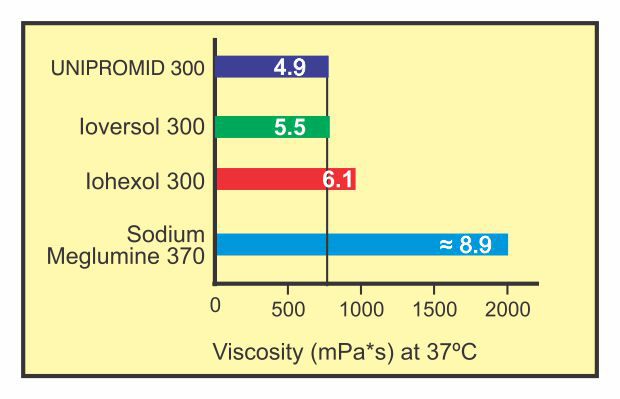
Lesser the viscosity in a contrast medium, leads to lesser haemodynamic changes. Low viscosity ensures not only less pain and heat sensation but also ease of administration resulting in better patient compliance.
| INDICATION | CONCENTRATION | VESSEL VOLUME |
|---|---|---|
| Guideline for intra-arterial administration Adults: |
||
| Cerebral Arterigraphy | 300mg I/ml | Carotid arteries : 3 - 12 ml. Vertebral arteries : 4 - 12 ml. Aortic arch injection (4-vessel) : 20 - 50 ml. Not exceed 150 ml of cumulative dose. |
| Peripheral Arteriography | 300mg I/ml | Subclavian or femoral artery: 5 - 40 ml. Aortic bi function : 25 - 50 ml. Not to exceed 250 ml of cumulative dose. |
| Coronary rteriography and left ventriculography |
370 mg I/ml | Right or left coronary artery : 5 - 40 ml. Left ventricle : 30 - 60 ml. Not to exceed 225 ml of cumulative dose. |
| Guideline for intravenous administration: Adults: |
||
| Excretory Urography | 300 mg I/ml | 300 mg/kg, IV (with normal renal function). Not to exceed 100 ml cumulative dose. |
| Contrast computed tomography Head Body (bolus injection) Body (bolus injection) |
300 mg I/ml |
50 - 200 ml 20 - 200 ml 100 - 200 ml Not to exceed 200 ml cumulative dose. |
| Contrast computed tomography Head Body (bolus injection) Body (bolus injection) |
300 mg I/ml |
41 - 162 ml 41 - 162 ml 81 - 162 ml Not to exceed 162 ml cumulative dose. |
| For pediatric patients: | ||
| Cardiac chambers and related arteries | 370 mg I/ml | <2 years : Safety and efficacy not established. >2 years : Inject 1 to 2 ml/kg intra-arterial. Not to exceed cumulative dose of 4 ml/kg. |
| Cantrast computerized tomography | 300 mg I/ml | <2 years : Safety and efficacy not established. >2 years : Inject 1 to 2 ml/kg IV. Not to exceed cumulative dose of 3 ml/kg. |
| Excretory Urography | 300 mg I/ml | <2 years : Safety and efficacy not established. >2 years : Inject 1 to 2 ml/kg IV. Not to exceed cumulative dose of 3 ml/kg. |
DOSAGE AND ADMINISTRATION:
General:
It is desirable that solution of radiopaque diagnostic agents for intravascular use be at body temperature when injected. In the event that crystallization of the medium has occured, place the vial in hot (60℃ - 100℃) water for about five minutes, then shake gently to obtain a clear solution. Cool to body temperature before use. Discard vial without use if solids persist. Patients should be well hydrated prior to and following UNIPROMID administration.
CONTRAINDICATIONS:
Do not administer Iopromde injection intrathecally. Inadvertent intrathecal administration may cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia and brain edema.
Preparatory dehydration (for example, prolonged fasting and the administration of a laxative) before UNIPROMID injection is contraindicated in pediatric patients because of risk of acute renal failure.
HOW SUPPLIED:
Single dose vials of 50ml and 100ml.
STORAGE:
UNIPROMID should be stored according to instruction on the label, protected from light and store at comtrolled room temperature.
Discard the unused portion.
